 by Ted Beyer
by Ted Beyer
We love our customers, and not just because they help keep the lights on! Educational Innovations’ customers share amazing things with us all the time. In fact, some of our best products have been created as a result of our customers’ discoveries, suggestions, or requests.
Recently, we received a comment on our website regarding our color-changing goldenrod paper. It prompted us to try a brand-new experiment that we think you’ll enjoy.
 Now, let me jump in here and tell you how proud we are to have what we believe to be the only remaining supply of color-changing goldenrod paper in existence. Teachers have treasured this stuff for decades. When the supply dried up, it was universally mourned.
Now, let me jump in here and tell you how proud we are to have what we believe to be the only remaining supply of color-changing goldenrod paper in existence. Teachers have treasured this stuff for decades. When the supply dried up, it was universally mourned.
Wait… what do you mean you don’t know about goldenrod paper? Really? Are you kidding?
Okay, let me back up then…
Back in the day…
Color-changing goldenrod paper has always been made with a dye that, when exposed to a base, changes from bright yellow to blood red.
Read all about it here, and click on the video below to see this stuff in action.
But then…
Several years ago, pretty much the entire paper industry phased out that particular dye for a number of reasons. Gone were the days when you could you run down to the office supply store and buy big sheets of this inexpensive acid base indicator. To make a long and very involved story short, after five years of searching, we finally managed to find what we think is the only source left on the planet. My point is: we have it!
And so, here we are!
Now back to this cool new experiment…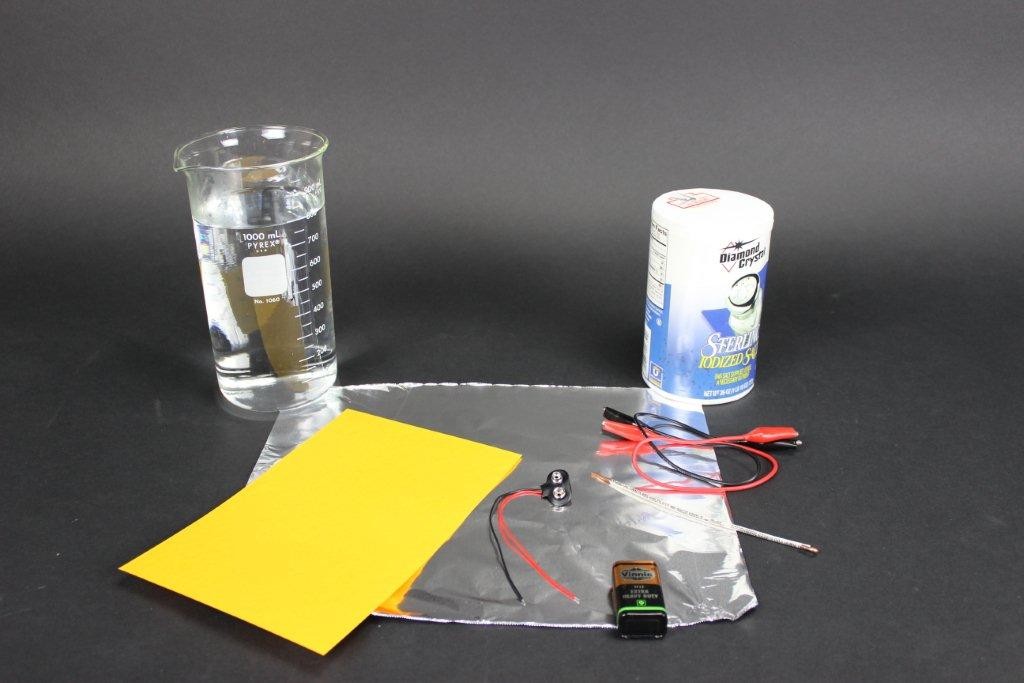
The set-up is fairly simple. You will need:
- a sheet of goldenrod paper
- table salt
- water
- a 9v or 12v DC source (a 9v battery works fine for this)
- a sheet of aluminum foil
- some hook up wire (I found that a couple of alligator clips and a nail or short length of wire came in handy)
Once you have all that together, it’s pretty simple. In a container, mix salt and warm water to create a saturated solution. (You need not be exact; I just dumped the salt into the water until it stopped dissolving. Then I added a bit more for good measure.) A tall container works well, but you can also use an edged cookie sheet.
 Dampen the entire sheet of goldenrod paper with the salt water (both sides). Then spread the paper out flat on a sheet of aluminum foil that is slightly bigger than your paper.
Dampen the entire sheet of goldenrod paper with the salt water (both sides). Then spread the paper out flat on a sheet of aluminum foil that is slightly bigger than your paper.
Connect the positive side of the DC source to the foil (the alligator clips come in handy here).
NOTE: Polarity does count on this one, so make sure you have connected it the right way around.
Connect the negative side of the DC source to the wire or nail (or other conductive material).
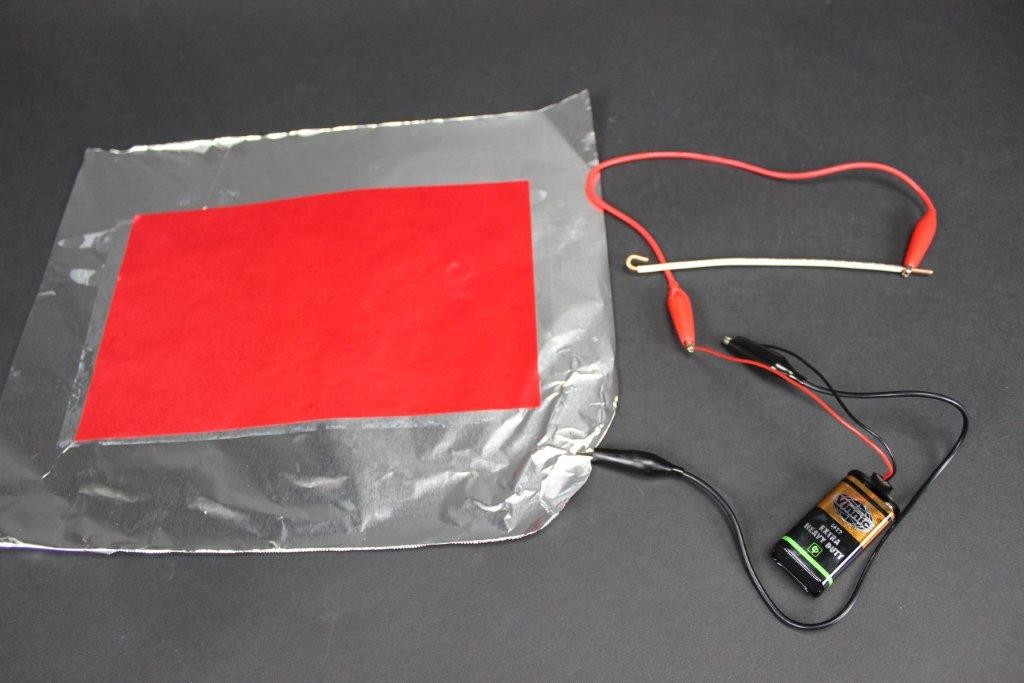
Touch the negative side wire to the paper and—MAGIC!—it will leave a bright red trail behind.
Like this:
In effect you are writing with electricity! How is this possible? The answer is simpler than you might think: it’s electrolysis of water.
Let’s look at the process:
Negative electrons come out of the negative terminal of the power source and react with the H+ of the HOH, producing hydrogen gas leaving OH-, which is basic, and thus turns our yellow goldenrod paper red.
At another place, to complete the circuit, OH- from water molecules give up their electrons which travel into the positive terminal of the battery and leaving H+ in the water (an acid), oxygen gas, and HOH. Around the positive terminal, then, we have an acid solution that maintains our goldenrod paper’s original yellow color.
Here’s an additional step, if you’re inclined to continue the experiment. If you wet the goldenrod paper with a weak washing soda or baking soda solution, the paper should dry red. Allow it to dry completely.
Then, if you wet the paper with water and place it on a piece of aluminum foil connected to the negative terminal of the battery, you should be able to change portions of the red paper to yellow by using the wire connected to the positive terminal of the battery. While salt water is not required for this step (baking soda and washing soda both contain sodium carbonate, which is a salt, after all), adding some NaCl does enhance the effect.
When water (HOH) is added to sodium carbonate (Na2CO3), one gets OH- ions (a strong base, Na+ and OH-, which turns goldenrod red) and H2CO3, which is a weak acid so it does not turn the paper yellow.
In a nutshell:
strong > weak
When the positive terminal is touched to the paper, the charge on the OH- goes into the positive terminal of the battery removing the base, so the paper turns yellow at that spot. Oxygen and water are also produced.
Of course, the whole process immediately stops unless electrons from the negative terminal of the battery is allowed to go into the water somewhere, which will react with the H+ leaving OH- (a basic solution at that point). Salt in the water is necessary to allow the positive charge and negative charge to more easily be the same so that the solution is always electrically neutral in charge.
Keep in mind that things always have to occur at the ends of the two metal leads. Why? Because “electrons cannot swim” as one of Educational Innovations’ founders, Ron Perkins, was fond of saying.
If you do not allow the paper to dry completely before re-wetting it, the newly transformed yellow, while observable, will almost instantly revert to red as the basic solution flows back to the newly yellowed area. While it does technically work, it’s not a very pretty or dramatic demonstration.
And one more thing…
Here’s one more thing to try if you have access to the material:
Instead of table salt, make a solution using sodium nitrate (NaNO3)—the reaction should be even faster, as it will make the water into an even better conductor. Sodium nitrate is more soluble in water than sodium chloride. This means that more NaNO3 will dissolve in the water.
Admittedly, there is a lot of science here. (Hope your eyes didn’t glaze over too much.) The real takeaway is that goldenrod, salt water and a little electricity make a really stunning demonstration of electrochemistry—an often overlooked area of science. And if you just forget about the science entirely, it’s still spectacular.
Or as we like to say, Super! Wow! Neat!®
 This demonstration is really a great deal of fun. To make it as easy as possible to jump right in, we’ve created a Goldenrod Electrochemistry Kit with (almost) everything you need. Just add aluminum foil, table salt and water. Oh, and don’t forget the color-changing goldenrod paper!
This demonstration is really a great deal of fun. To make it as easy as possible to jump right in, we’ve created a Goldenrod Electrochemistry Kit with (almost) everything you need. Just add aluminum foil, table salt and water. Oh, and don’t forget the color-changing goldenrod paper!

If I wet the paper with ammonia, and allow it to air dry, will the original color return?
Hi Nick, it should return back to its original color!