 by Dr. Kenneth Lyle
by Dr. Kenneth Lyle
The demonstration
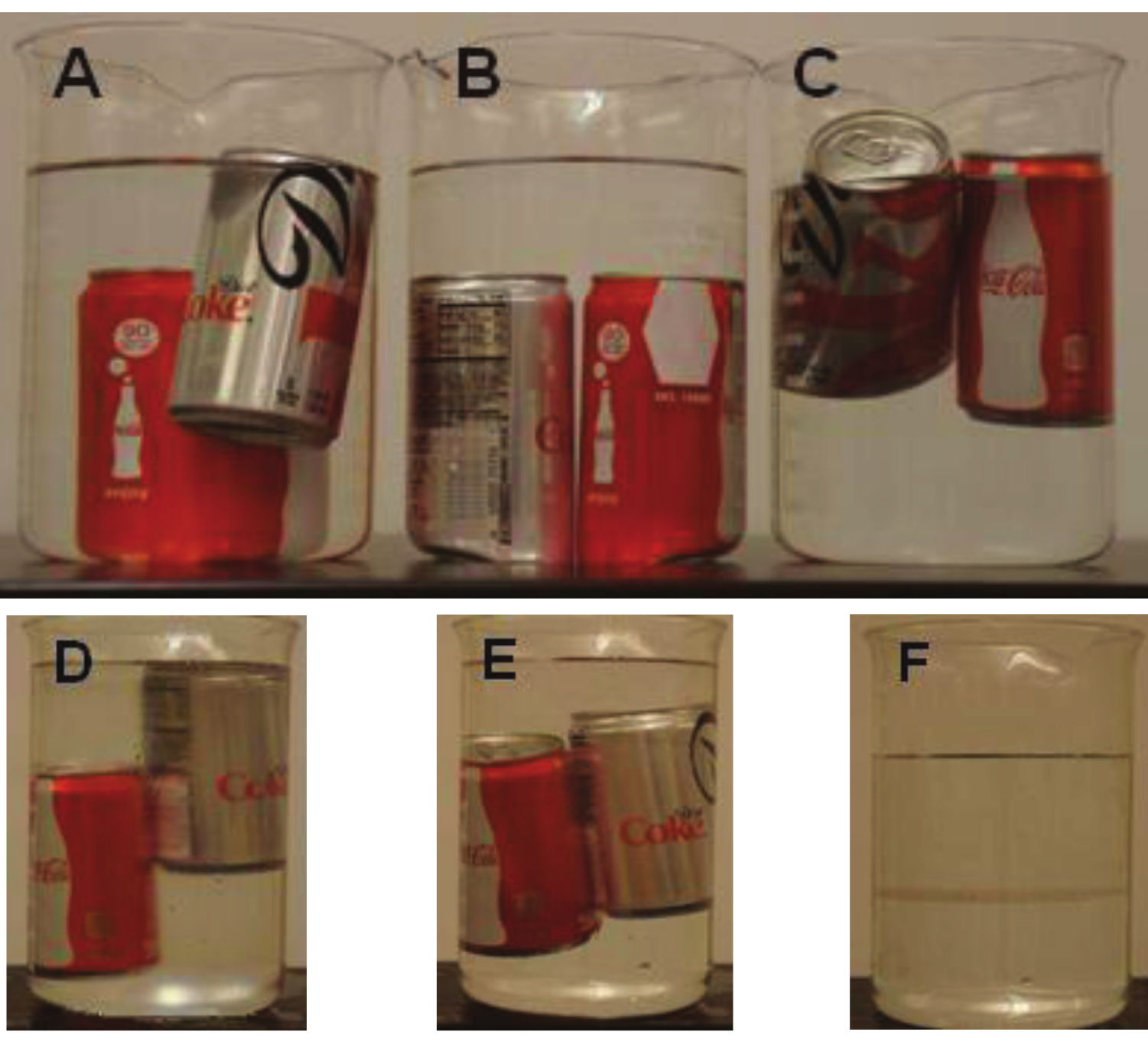
The Poly Density Bottle is a fascinating demonstration primarily due to the phenomena being counterintuitive to what one would expect. The bottle containing white and blue beads suspended in a clear and colorless liquid is shaken vigorously, distributing the beads randomly throughout (bottle A). Upon standing, the beads separate from one another (bottle B) with the white rising to the surface while the blue sink to the bottom (bottle C). Then, the two sets of beads move towards one another (bottle D) meeting near the middle (bottle E). This demonstration can be easily repeated again and again. And, once prepared, it can be stored for subsequent use year after year. No additional preparation is required.
The explanation
The underlying chemical concepts associated with this demonstration are: 1) relative densities of solids, liquids and solutions, 2) miscibility of liquids, and 3) how the miscibility can be affected by the addition of salt.
The liquid in the Poly Density Bottle is a solution of water, isopropanol and sodium chloride. Isopropanol is less dense than water. Water and isopropanol are completely miscible. However, when salt is added to the mixture, the water preferentially interacts with the sodium and chloride ions “letting go” of the isopropanol molecules. Being less dense than the salt water, the isopropanol separates and forms a layer on top of the salt water.
The relative densities of all materials in the bottle are important for understanding the demonstration. In order of decreasing density:
Salt Water > Blue Beads > White Beads > Isopropanol
Shaking the bottle not only randomly distributes the beads but also creates a pseudo-homogeneous solution with a density that lies between those of the two different colored beads. Thus the blue beads sink to the bottom while the white beads rise to the top upon standing. The salt water and isopropanol begin to separate with the salt water moving downwards while the isopropanol moves upwards. The blue beads, being less dense, rise as the salt water collects at the bottom, and the white beads, being denser than the isopropanol, sink.
Demystifying the Poly Density Bottle
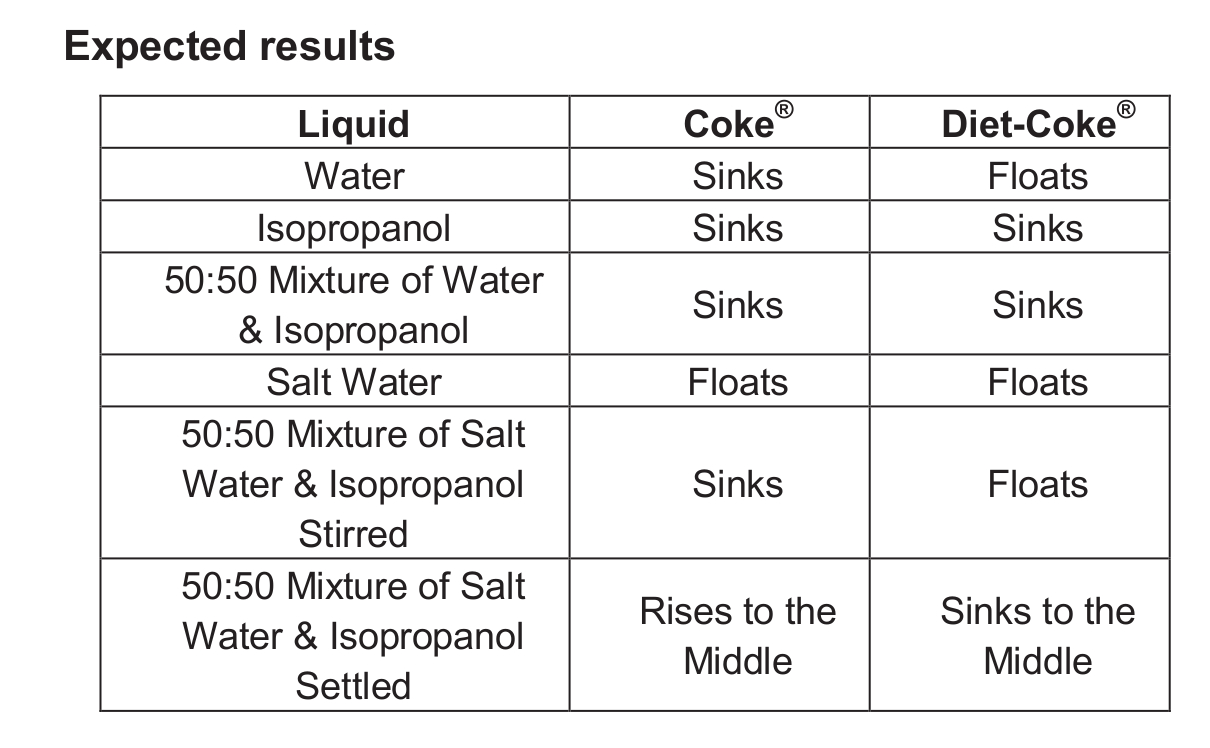
To help students understand this phenomenon, I use a modified version of the Sink-Float Coke® demonstration, which involves placing the cans of soda in water. The regular Coke® sinks while the Diet-Coke® floats due to differences in relative densities. The following is a description of how one can use the sodas to explain the Poly Density Bottle demonstration.
Materials
- 8-oz cans of Coke® and Diet-Coke® (one of each minimum)
- Tap water
- Isopropanol ~ 600 mL (I use pure isopropanol; if you use drug store variety, be sure to use 91% or higher.)
- Kosher salt ~150 grams (Kosher salt will not cloud up the water when dissolved in it.)
- Large plastic mixing spoon
- At least two 1-L beakers (I prefer to use the tall form, especially for the final part of this demonstration. For the purposes of this article, I used a 2-L beaker.)
- Plastic cafeteria tray to catch spills
Safety
- Wear safety glasses/goggles while performing this demonstration.
- Wear protective gloves when working with the isopropanol.
- Perform demonstration in a well-ventilated area to avoid inhalation of the isopropanol vapors.
- Isopropanol is flammable; check the area for, and remove any sources of, ignition before using.
- Empty the soda cans when finished to prevent anyone from drinking from them.
Advanced preparation
This demonstration can be assembled to use as a kit year after year and doing so eliminates issues concerning the disposal of isopropanol. The five liquids/solutions can be prepared and then stored in separate designated bottles. To prevent cross contamination, use separate sets of beakers and sodas for each liquid/solution tested.
Procedure
The basic procedure is the same for each of the following five combinations of liquids:
1. Water
2. Isopropanol
3. 50:50 mixture of water and isopropanol
4. Salt water
5. 50:50 mixture of salt water and isopropanol
- Pour about 600 mL of liquid to be tested into a clean dry 1-L beaker.
- Carefully place the regular can of Coke® into the liquid. Be sure that an air bubble is not trapped underneath the can.
- Note whether the can floats or sinks.
- Transfer the liquid to a second clean dry 1-L beaker.
- Carefully place the can of Diet-Coke® into the liquid. Be sure that an air bubble is not trapped underneath the can.
- Note whether the can floats or sinks.
- For the 50:50 mixture of salt water and isopropanol, stir the mixture vigorously before adding the can of soda. Then allow time for the mixture to separate.
- For the final step (after discussion), pour the mixture into the empty beaker, stir vigorously, and then allow it to sit undisturbed. The interface between the salt water and the isopropanol becomes visible.
B Density compared to isopropanol
C Density compared to salt water
D Salt water and isopropanol mixed thoroughly
E Isopropanol and salt water separate creating an interface where the cans come to rest
F When the mixture of isopropanol and salt water is left standing, the interface is clearly visible
Disposal
Empty out the cans of soda used in the demonstration, rinse the outside and place in a designated recycling bin. The isopropanol should not be poured down the drain. Consult local regulations for appropriate means of disposal.
Resources
The Poly Density Kit can be purchased from Educational Innovations, Inc.
Dr. Kenneth Lyle is a lecturing-fellow in the Department of Chemistry at Duke University. Dr. Lyle instructs the CHEM 180 Chemistry Outreach Service Learning Course.
Reprinted with permission from the March 2013 edition of CHEM 13 NEWS. For more great chemistry experiments and activities, visit Chem13 News.

We have a problem. The regular Coke no longer floats. Coca Cola has gone from 8 oz to 7.5oz but it looks like they kept the can the same. There is an air gap and the coke doesn’t completly sink, it stays just barely at water level. I will try to see if the 12oz can can work and sink.
Well, that would certainly stink! I guess that extra .5 oz they don’t put in the can saves Coke a boatload of money while messing up a great science activity!