Using real world examples is a great way to help students understand abstract ideas. These discussion starters will help you set up a unit on density.
Using real world examples is a great way to help students understand abstract ideas. These discussion starters will help you set up a unit on density.
Density Basics
These basic questions will get you started on your discussion of D = M ÷ V.
- What is the definition of mass?
- What tools are used to measure it?
- What units are used to measure it?
- What is the definition of volume?
- What tools are used to measure it?
- What units are used to measure it?
- What is the definition of density?
- What two measurements must be taken to calculate it?
- What units are used to measure it?
Density in Real Life Situations
Q: Why do you have to shake salad dressing before you use it?
A: Because the liquids are not of the same density and are imiscible, they separate upon standing based on their relative densities. Shaking the salad dressing helps blend the various liquids together temporarily.
Q: In general, most compounds become denser as they transition from liquids to solids, because their molecules are more closely packed. The solid compound will sink in the liquid compound, because it is denser. Ice, however, doesn’t abide by this rule—it floats on water. Why do you think this is?
A: Water molecules are hydrogen bonded to 4 other water molecules in ice. This traps air between its molecules as it freezes, decreasing the overall density of the ice. Water molecules are only hydrogen bonded to 3.4 other water molecules in liquid water, so they are closer together.
Q: Body mass index (BMI) is a measurement that compares a person’s height (as an estimate of volume) and weight to determine whether they are within a healthy weight range. However, athletes tend to have higher BMIs than nonathletes of the same size. Why do you think this is?
A: Muscle is denser than fat, so people with a higher proportion of muscle to fat weigh more, despite being the same size.
Q: Oil is typically less dense than water. During an oil spill, specialized boats called oil skimmers are deployed to help clean-up efforts. Based on your knowledge of the behavior of liquids of different densities, what do you think an oil skimmer does?
A: Some oil floats to the surface of the water. Oil skimmers scoop up the oil off the surface.
Q: Assume you have a one pound sponge. If you melt it down to a plastic soup with all the bubbles gone, what will happen to the mass, volume and density?
A: It will be much smaller (volume decreases), but it will still weigh one pound (mass unchanged). However, it is now denser than when it was a sponge.
Q: A U.S. aircraft carrier weighs more than 200,000,000 pounds, yet it can float. Why?
A: Because its weight is spread out over a great distance (increased volume) and because its hull has air inside.
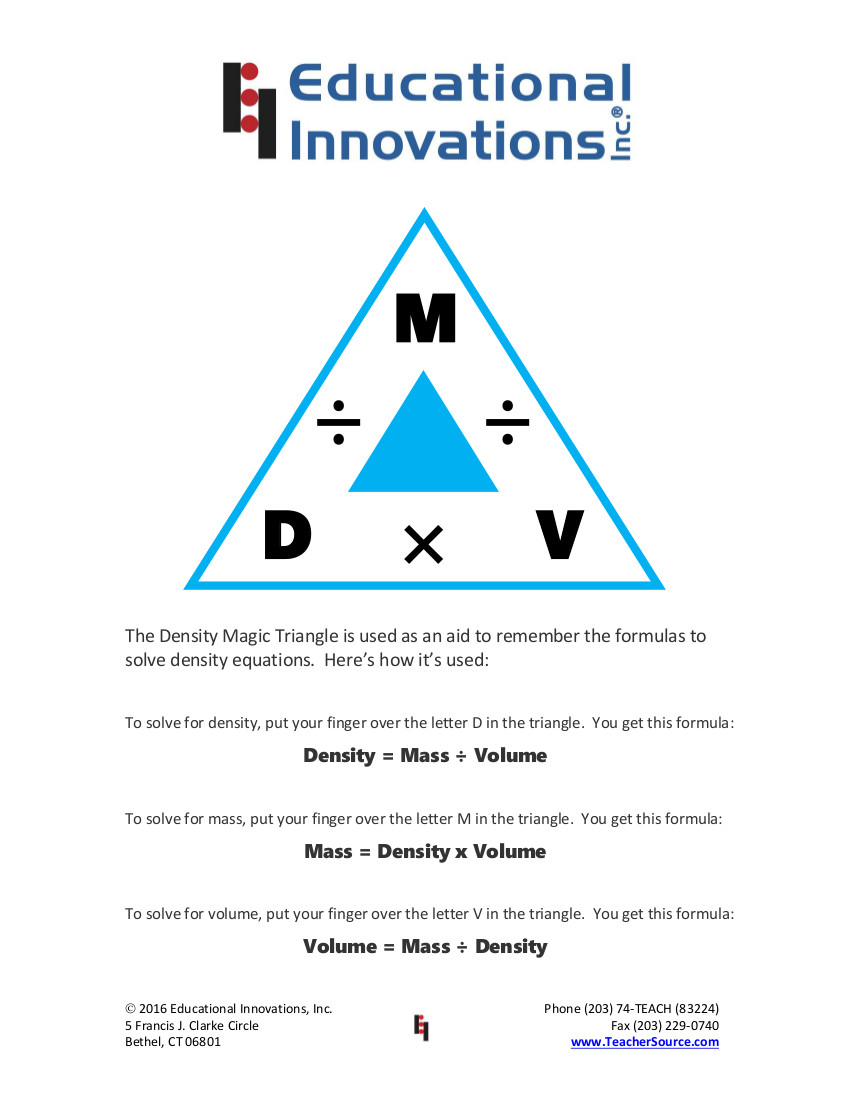
The Density Magic Triangle
The Density Magic Triangle is used as an aid to remember the formulas to solve density equations. When beginning a unit on density, it is helpful to place this on the whiteboard or at the top of a worksheet so your students can access the information in a tangible manner.
Click on the image below for a printable PDF version of this instructional sheet.