 by: Tami O’Connor
by: Tami O’Connor
One of my all time favorite air pressure activities is an oldie and a goodie! It involves getting an egg into a classic, hard-to-find milk bottle, like the ones  delivered to grandma’s door. Unfortunately, some students (and some teachers) still think an egg can actually be sucked into a bottle. As you probably know because the air pressure is greater outside of the bottle than inside, the better explanation is that the egg is literally pushed into the milk bottle.
delivered to grandma’s door. Unfortunately, some students (and some teachers) still think an egg can actually be sucked into a bottle. As you probably know because the air pressure is greater outside of the bottle than inside, the better explanation is that the egg is literally pushed into the milk bottle.
Here is the explanation… The milk bottle and egg demo begins by placing two or three burning matches or a burning strip of paper into the empty bottle. Then a shelled, moistened hard-boiled egg is placed on the mouth of the bottle. The egg is clearly larger than the opening in the bottle. The air inside the bottle begins to heat up and subsequently expands. It is easy to notice the egg dancing around a bit as the air inside the bottle escapes around it.
Shortly after the flame inside the bottle extinguishes, the egg enters the bottle with a noticeable pop! There is no doubt that the kids just love this demonstration. I’ve done it at a number of science demonstrations and assemblies, as well as in my own classroom, and the response is always a surprised gasp followed by applause!
The only thing the kids enjoy more than watching the egg enter the bottle is watching me trying to get the egg back out of the bottle… In my younger days I learned that I could only do that demo once since I had to wait to get the kids out of my class before I would attempt to blow the egg out of the bottle. I’ve had egg on my face more than once… The trick is, after blowing into the mouth of the inverted bottle, moving your face away quickly… well, very quickly.
As I got more creative (and spoke with more experienced teachers), I realized that there are other ways of getting the egg out of the bottle besides blowing into the mouth. Another way to accomplish the same results (while remaining clean!) is to pour hot water on the outside of the bottle while the egg is seated the neck of the inverted bottle. I usually rinse the inside of the bottle out with cold water first. The process of running hot water over the outside of the bottle filled with cool air serves to warm the bottle, thus warming the air inside the bottle and causing the air to expand once again, forcing the egg out of the bottle. Blowing a hair dryer over the outside of the bottle achieves the same results.
If you want to merge two lessons into one, you can also use an Alka Seltzer tablet, or vinegar and baking soda, to generate Carbon Dioxide gas inside the bottle, and force the egg out by increasing the amount of gas inside the bottle.
Another common, but erroneous, explanation can be found on the web and even in some books. In fact, about half of the explanations on the web seem to use this explanation: that the burning material removes oxygen, thus lowering the pressure inside the bottle. This ignores the fact that, for each molecule of oxygen removed, a molecule of carbon dioxide or two molecules of carbon monoxide are formed.
Some students have argued that it’s gravity that pulls the egg inside the bottle… That’s questionable given that the egg is much larger than the mouth of the bottle, but one easy way to combat that question is actually my favorite way to demonstrate the concept of air pressure. This idea was shared with us by science teacher, Jeff Feidler of Ursuline Academy in Wilmington, DE.
Cut a small piece from the large end of the egg so that it stands easily. Place a birthday candle in the narrow part of the egg and ignite the candle. Lower the bottle onto the egg so that bottle touches the  surface of the egg. As the candle extinguishes, air pressure should be sufficient to allow the bottle to be lifted while the egg is hanging on. Due to the lower air pressure inside the bottle, the egg will remain in the opening of the bottle. Hold the bottle steady. The egg will eventually be pushed upward into the bottle. This version of the demonstration will take a little longer than the traditional method detailed above, but is a great way to celebrate birthdays in your classroom…..and to show that gravity is not the explanation!
surface of the egg. As the candle extinguishes, air pressure should be sufficient to allow the bottle to be lifted while the egg is hanging on. Due to the lower air pressure inside the bottle, the egg will remain in the opening of the bottle. Hold the bottle steady. The egg will eventually be pushed upward into the bottle. This version of the demonstration will take a little longer than the traditional method detailed above, but is a great way to celebrate birthdays in your classroom…..and to show that gravity is not the explanation!
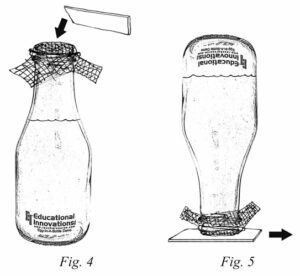
Educational Innovations sells these hard-to-find milk bottles for an additional activity that utilizes a one way mesh screen. Water can be poured into a bottle covered with a screen, and when the bottle is inverted, the water doesn’t come out!
Procedure:
1. With an elastic band attach a double layer of nylon net screen to the top of a milk bottle.
2. Show students that water can easily be poured into the bottle through the screen.
3. Place a small piece of card stock (ca. 7 x 7 cm; 3 x 3″) on top of the
screen, hold it in place with your hand, and invert the bottle over a sink or bowl.
4. Slowly slide the card out.
5. Ask students why you can you pour water into the bottle, but when inverted the water does not flow out?
6. Tip the inverted bottle slightly and then bring back to the upside down position. The water will begin to flow out of the bottle while it’s tilted and then will stop flowing when the bottle is back in the starting position.
Why does this happen? The force of flowing water allows the water to enter the bottle through the screen. Water in motion tends to remain in motion. When the bottle is inverted, the water stays in the bottle because the molecules of water have a greater attraction to themselves than to the screen. The water is said to exhibit surface tension. In addition, when the bottle is inverted, a small amount of water is lost from the bottle, the air which remains at the top of bottle slightly expands, and the pressure of the air inside the bottle is slightly less than the outside atmospheric pressure.
The combination of the water’s surface tension and the greater outside atmospheric pressure explains why the water tends to remain in the bottle. When the bottle is tipped slightly and then returned to the upright position, outside air enters the bottle and water runs out until the forces return to static equilibrium.
Whether you have your own bottle or choose to purchase one from Educational Innovations, you can have tons of science learning fun with your students in almost every grade level!

I love this demonstration.
You can also get the egg into the bottle without burning the paper. This avoids the messy charred paper etc. although the process takes longer.
Fill the bottle with very hot water and allow it to sit for a few minutes. Empty the water from the bottle and then place the peeled moist egg on the mouth. As the air in the bottle cools, the egg will be pushed into the bottle.
Great point! This is especially good if you have students perform this experiment in smaller lab groups rather than using it as a whole class demonstration!
Brilliantly explained. I performed the activity in the class room and my students loved it! Thanks